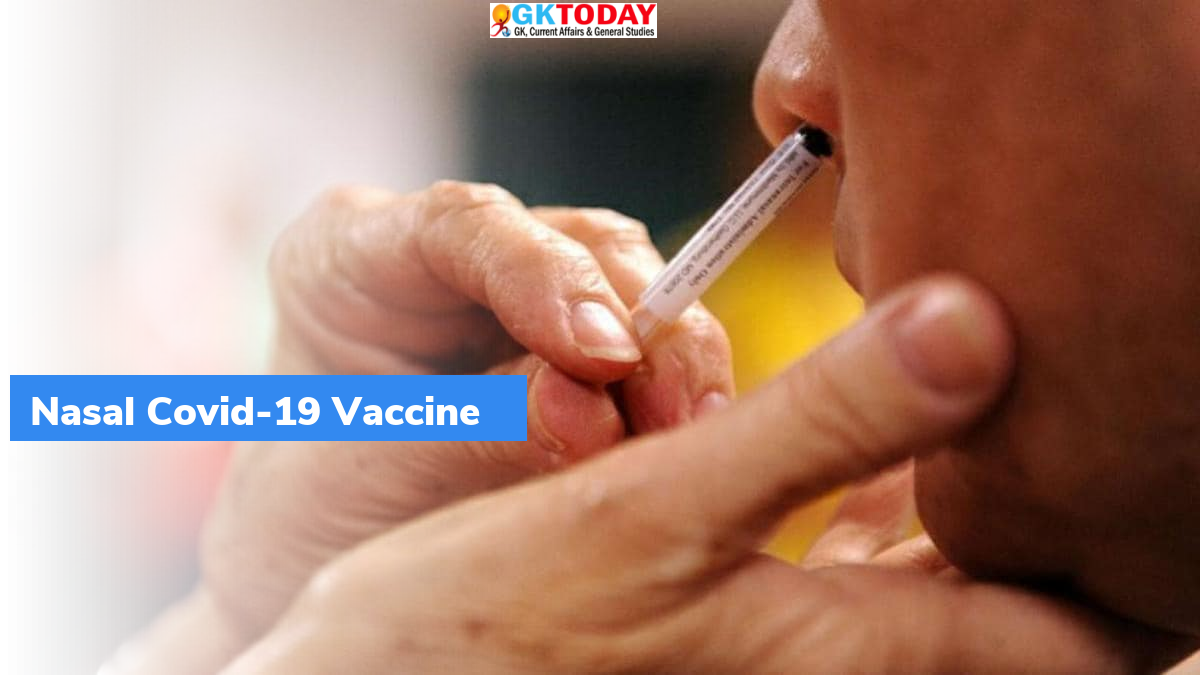
Bharat Biotech’s intranasal vaccine – Update
Bharat Biotech recently sought approval for its intranasal Covid vaccine candidate, BBV154, as two-dose vaccine as well as booster dose. It submitted data from Phase 3 clinical trials of BBV154 to seek approval.
Highlights:
- The heterologous booster infers that, third or subsequent dose of the vaccine is not similar to its primary dose.
- Usually, primary doses include two shots.
- According to Bharat Biotech, BBV154 is stable at 2-8 degrees Celsius. It is safe, well-tolerated and immunogenic under controlled clinical trials.
About BBV154:
- BBV154 is an intranasal vaccine, that have capability to produce local antibodies in upper respiratory tract. These are capable of reducing infection and transmission.
- To evaluate BBV154 as primary dose and booster dose, two separate & simultaneous clinical trials were conducted.
- Primary dose Phase III trials were conducted on more than 3000 individuals, to test its safety, and immunogenicity. Trials were conducted across 14 sites in India.
- On the other hand, studies on heterologous booster dose were conducted on over 800 individuals.
- This vaccine has been formulated to allow its delivery through nasal.
- Nasal delivery system is cost-effective for low and middle-income countries.
- BBV154 nasal vaccine has been developed by Bharat Biotech, in association with the Washington University.
Bharat Biotech International Limited (BBIL):
BBIL is headquartered in Hyderabad. The company is engaged in discovering drug, developing drug, manufacturing vaccines, pharmaceuticals and health care products. It was established in 1996.
Month: Current Affairs - August, 2022
Category: Science & Technology Current Affairs