Asymmetric Organocatalysis
This year’s Nobel Prize in chemistry has been won by two organic chemists who developed asymmetric organocatalysis more than two decades ago by David MacMillan of Princeton University, USA and Benjamin List of the Max Planck Institute for Coal Research, Germany. The work done by them has shown that small simple organic molecules with enzymes and metal complexes can compete and win at their own game: catalyzing reactions to produce chiral molecules.
What is catalysis?
- According to an estimate made in 2015, catalysis accounts for 35% of world GDP. Catalysts have fundamentally changed the way we understand and apply chemistry.
- In catalysis, the focus is not only on speeding up the reactions, but also on performing enantioselective or asymmetric reactions, those that only produce a mirror image (enantiomer) of the transferred molecules.
- Since certain biological molecules, amino acids and sugars, only exist as individual enantiomers, our body has an innate ability to differentiate between enantiomers.
- This means that the same molecule depending on its handedness can smell like lemon or orange, and often only one enantiomer of an active ingredient molecule has a positive effect, while the other may even be harmful or does nothing.
- Enzymes cannot normally be produced in the laboratory, but must be isolated from biological sources.
- This year’s winners showed that even small and simple chiral compounds can catalyze complex reactions just as well or sometimes even better than enzymes or metals.
- Organocatalysts are easy to manufacture and often cheap, hence, they have the potential to make the various synthetic routes more environmentally friendly.
About Asymmetric Organocatalysis
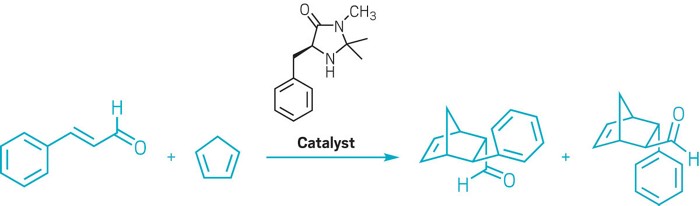
At the beginning of the 21st century, MacMillan and List to bring about asymmetric reactions started to explore using small organic molecules. After working on redesigning antibodies for chemical catalysis, List then decided to check what happens after reducing biocatalysts to their most basic chemical form. The enzyme aldolase A carries out aldol reactions in the body, in which two carbonyl compounds combine to form a new carbon-carbon bond. But in reality, only three amino acids are responsible for its catalytic activity. In 2000, List and his team discovered that one of them, proline, can only catalyze asymmetric aldol reactions with enantiomeric ratios up to 98: 2. Meanwhile, MacMillan wanted to develop a catalyst for the Diels-Alder reaction that could overcome the disadvantages of metal catalysts highly sensitive to moisture and air. His team discovered that a modified phenylalanine can catalyze asymmetric reactions between α, β-unsaturated dienes and aldehydes with enantiomeric ratios up to 98: 2. Organocatalysts bind to reactive molecules and form short-lived intermediates that are more reactive than substrate molecules alone. Because the catalyst is chiral, the catalyst transfers its workability to the substrate, thereby controlling which side of the intermediate can react the most.
Conclusion
The field has come a long way from its humble beginnings. The winners’ surprise discovery that small organic molecules can catalyze complex enantioselective reactions, and the simple explanations for them, sparked an explosion of research in the field.