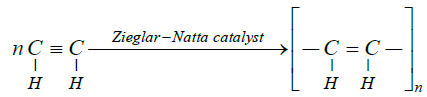I. It is used in welding industry.
II. It is raw material for preparing plastics.
III. It is easily obtained by mixing silicon carbide and water.
Of these statements :
Answer: I and II are correct
Notes: Welding refers to the process of joining two or more metals together. Approximately 20% of acetylene is consumed for oxyactylene gas welding and cutting due to high temperature of flame. Combustion of acetylene with oxygen produces a flame of over 3600 K (3300°C, 6000°F). Oxyacetylene is the hottest burning common fuel gas. Oxyacetylene welding was a very popular welding process in previous decades; however the development and advantages of arcbased welding process have made oxy fuel welding nearly extinct. This high temperature of flame makes head of metal pieces to be joined melt and they joined together when solidified on cooling. Following is the chemical reaction occurring in above process: 2C2H2 + 5O2 - 4CO2 + 2H2O. The polymerization of acetylene with Ziegler – Natta catalyst produces polyacetylene films. Polyacetylene, a chain of CH centres with alternating single and double bonds, was the one of first discovered organic semiconductors. Chemical equation representing polymerization of acetylene: